In today’s aging societies, diseases affecting the bones and joints are becoming increasingly common. For example, in Japan alone, nearly 13 million people suffer from osteoporosis, a condition that severely weakens bones and makes them fragile. If we are to find effective treatments for such disorders, understanding the cellular processes involved in the maintenance of bone and joint tissue is an essential first step.
Osteoclasts are a particularly important type of cell involved in bone maintenance. These cells absorb old or damaged bone and digest it, allowing the body to reuse important materials like calcium and giving way to new bones. As one might expect, various bone diseases arise when osteoclasts do not fulfill their role properly. Scientists have been investigating the mechanisms that regulate the proliferation and differentiation of precursor cells into osteoclasts.
In a study published in 2020, researchers from Tokyo University of Science (TUS) led by Professor Tadayoshi Hayata revealed that the cytoplasmic polyadenylation element-binding protein 4 (Cpeb4) is essential in osteoclast differentiation. (Differentiation is the process by which cells develop into particular cell types, such as osteoclasts.) The researchers also discovered that this protein, which regulates the stability and translation of messenger RNA (mRNA) molecules, transported into specific structures within the nucleus of the cell when osteoclast differentiation was induced. However, just how this relocation occurs and what Cpeb4 exactly does within these nuclear structures still remains a mystery.
Now, in a recent study published in the Journal of Cellular Physiology on January 29, 2024, Hayata and Yasuhiro Arasaki, also from from TUS, tackled these knowledge gaps. Interested in the intricate and complex process of osteoclast differentiation, they sought to more thoroughly understand how the “life cycle” of mRNA, i.e., mRNA metabolism, is involved.
How was the study conducted?
First, the researchers introduced strategic modifications into Cpeb4 proteins and performed a series of experiments in cell cultures. They found that the localization of Cpbe4 in the abovementioned nuclear bodies occurred owing to its ability to bind to RNA molecules.
Afterwards, seeking to understand the role of Cpeb4 in the nucleus, the researchers demonstrated that Cpeb4 co-localized with certain mRNA splicing factors. These proteins are involved in the process of mRNA splicing, which is a key step in mRNA metabolism. Put simply, it enables a cell to produce diverse mature mRNA molecules (and eventually proteins) from a single gene.
Through RNA sequencing and gene analysis in Cpeb4-depleted cells, the researchers found that Cpeb4 alters the expression of multiple genes associated with splicing events in freshly differentiated osteoclasts.
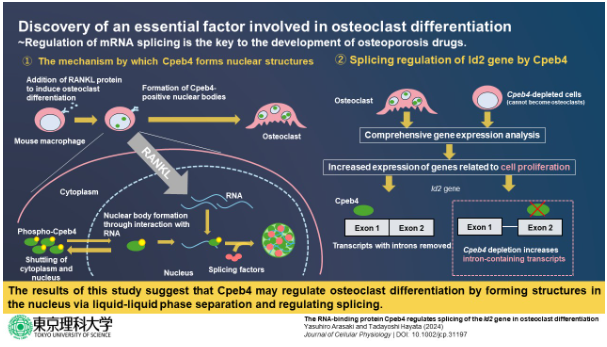
Finally, through further experiments, they concluded that Cpeb4 only altered the splicing patterns of Id2 mRNA, an important protein known to regulate osteoclast differentiation and development.
What is the significance of these findings?
Overall, this study sheds important light on the mechanisms that regulate osteoclast differentiation. “Through this research, we were able to identify important factors involved in regulating mRNA splicing during the osteoclast differentiation process and obtained new knowledge regarding the control of mRNA splicing during osteoclast differentiation,” Hayata commented.
While the contribution of Cpeb4 is smaller than that of RANKL, a signaling factor that induces osteoclast differentiation, targeting Cpeb4 may have the advantage of reducing the side effects of existing drugs as excessive inhibition of osteoclast differentiation with RANKL inhibitory antibodies would halt bone remodeling.
Importantly, the results contribute to a more detailed understanding of how bones are maintained. “Although we used cultured mouse cells in our study, there are also research reports that show a correlation between variations in the Cpeb4 gene and bone density in humans,” Hayata said. “We hope that our findings will help clarify the relationship between these two in the near future.”
Most importantly, the findings of the present study may prove to be a crucial stepping stone for advancing diagnostic techniques and treatments for bone and joint diseases. Use of genome-wide association study has evidenced a correlation between single nucleotide polymorphisms in introns of the Cpeb4 gene region and the estimated bone density. Therefore, it is possible that Cpeb4 expression and activity can be used as diagnostic criteria.
However, the researchers note that it is unclear whether Cpeb4 actually regulates bone metabolism in vivo. Therefore, clarification of the molecular basis of Cpeb4 in bone metabolism in mice would help to establish a therapeutic approach. Additionally, recent studies have reported that Cpeb4 is expressed in various cancer cells and contributes to cancer cell survival. In cancer, Cpeb4 contributes to mRNA stability, although splicing regulation may exist.
“The discovery of part of the mechanisms by which Cpeb4 controls osteoclast differentiation could lead to the elucidation of pathologies, including osteoporosis and rheumatoid arthritis, and ultimately become the foundation for the development of new therapeutic drugs,” a hopeful Hayata concluded.
We too hope these efforts will pave the way for a brighter future for the millions of people suffering from osteoporosis and similar disorders, enabling them to live more active and fulfilling lives.
For more information, you can read the original paper here.
The views expressed in this article are the author’s own and do not necessarily reflect Fair Observer’s editorial policy.
Support Fair Observer
We rely on your support for our independence, diversity and quality.
For more than 10 years, Fair Observer has been free, fair and independent. No billionaire owns us, no advertisers control us. We are a reader-supported nonprofit. Unlike many other publications, we keep our content free for readers regardless of where they live or whether they can afford to pay. We have no paywalls and no ads.
In the post-truth era of fake news, echo chambers and filter bubbles, we publish a plurality of perspectives from around the world. Anyone can publish with us, but everyone goes through a rigorous editorial process. So, you get fact-checked, well-reasoned content instead of noise.
We publish 2,500+ voices from 90+ countries. We also conduct education and training programs
on subjects ranging from digital media and journalism to writing and critical thinking. This
doesn’t come cheap. Servers, editors, trainers and web developers cost
money.
Please consider supporting us on a regular basis as a recurring donor or a
sustaining member.
Will you support FO’s journalism?
We rely on your support for our independence, diversity and quality.







Comment